Recently, Xi Chen, Associate Professor of the China-UK Low Carbon College at Shanghai Jiaotong University, with the team of Professor Ning Yan from National University of Singapore published an collaborative paper in the renowned journal Accounts of Chemical Research (IF =20.832) entitled Expanding the Boundary of Biorefinery: Organonitrogen Chemicals from Biomass.
In this Account, research group summarized their efforts to establish new reaction routes to synthesize valuable organonitrogen chemicals from renewable resources. Enabled by tailor-designed catalytic systems, diverse nitrogen-containing products including amines, amino acids, nitriles, and N-heterocycles have been obtained from a range of biomass feedstock either directly or via intermediate platform compounds. Two strategies to produce organonitrogen chemicals are presented. The first strategy is preparing nitrogen-containing chemicals through integrating ammonia to lignocellulose or waste oil biomass. The second strategy involves the use of nitrogen-containing biomass chitin and chitin derivatives as the starting materials. Based on these two strategies, the catalyst design methods for different biomass raw materials and target products are systematically discussed, the relationship between catalyst structure and activity is discussed, and the reaction path and mechanism are expounded.
Xi Chen and Song Song (Associate Professor at Tianjin University) are the co-first authors, Professor Ning Yan Ning is the correspondence author, and Shanghai Jiao Tong University is the first affiliation of this article.

This article was selected as the Supplementary Cover
Background
Organonitrogen chemicals are essential in many aspects of modern life. Over 80% of the top 200 prescribed pharmaceutical products contain at least one nitrogen atom in the molecule, while all top 10 agrochemicals contain nitrogen, just to name a few. At present, the prevailing industrial processes for manufacturing organonitrogen chemicals start from nonrenewable fossil resources, but eventually we have to make these chemicals in a more sustainable manner. Biomass represents the largest renewable carbon resource on earth, which is inexpensive and widely available. Integrating biomass into the organonitrogen chemical supply chain will mitigate the carbon footprint, diversify the product stream, and enhance the economic competitiveness of biorefinery.
In this Account, two strategies were summarized to synthesize value-added organonitrogen chemicals from renewable resources. The first is to use renewable biomass feedstocks as carbon sources to replace fossil resources. With the development of a biomass refinery, various intermediate chemicals have been generated, providing a large chemical library to access the desired organonitrogen compounds by incorporating N-functional groups using NH3. The second strategy involves the use of chitin, the most abundant nitrogen-containing biopolymer on earth, as starting material to produce organonitrogen chemicals. By exploiting tailor-designed catalytic protocols, a few dozen valuable organonitrogen products including amines, amino acids, nitriles, and N-heterocyclic compounds have been synthesized from cellulose, hemicellulose, lipids, chitin, and their derivatives.
Introduction
Industrial preparation of such chemicals mainly through the refining of fossil resources to obtain intermediates, and then coupled with ammonia or other nitrogen sources derived from ammonia to prepare different nitrogen-containing chemicals (Figure 1). The method has the problems such as high carbon emission, high energy consumption, low efficiency and unsustainable.
Figure 1. Prevailing industrial processes to synthesize organonitrogen
chemicals from fossil resources.
In order to solve the above-mentioned problems, the research group proposed to prepare value-added organonitrogen chemicals from renewable biomass (Figure 2). In the first strategy, lignocellulosic biomass or the derived platform compounds were integrated with ammonia as the main carbon source. In the second strategy, chitin is selected as a unique biomass resource, which serves as both the carbon and nitrogen sources to prepare a variety of organonitrogen chemicals without the addition of external nitrogen sources. By adopting the two strategies, a series of value-added organonitrogen chemicals were successfully prepared.
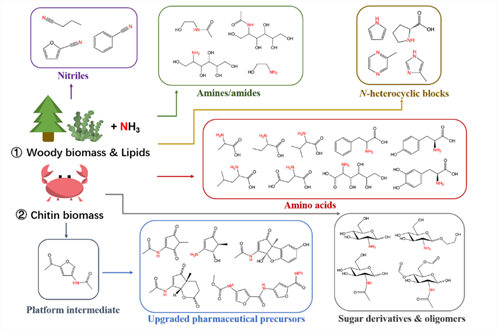
Figure 2. Overview of the two strategies to convert biomass into a broad range of organonitrogen chemicals.
There are a variety of raw lignocellulosic biomass and their derived platform chemicals which are suitable starting materials to produce diverse organonitrogens. Glycerol, as a byproduct of biodiesel refining, is also a suitable raw material for the preparation of organonitrogen chemicals. A series of biomass-derived α-hydroxyl acids have been converted to the corresponding α-amino acids by amination in NH3 aqueous solution under Ru/CNT catalysis. This study establishes a rapid chemical route to producing a series of amino acids from lignocellulosic biomass and paves the way for the chemical synthesis of proteins from agricultural waste in the future (Figure 3). Following this work, a mild photocatalytic system has been established to aminate glucose and the biomass-derived αhydroxyl acids to α-amino acids. The reaction temperature dramatically decreases from 220 °C in the thermal catalytic condition to only 50 °C, with no external reductant or sacrificial reagent needed. Different semiconductor materials were examined, and CdS performed the best under visible-light irradiation. Notably, the tuning of CdS morphology played a crucial role in the amino acid yield. Apart from glucose and biomass-derived α-hydroxyl acids, glycerol has been utilized as an inexpensive feedstock to synthesize alanine. A multifunctional catalytic system constituting a bimetallic Ru1Ni7/MgO catalyst and NaOH as an additive was established for the desired transformation
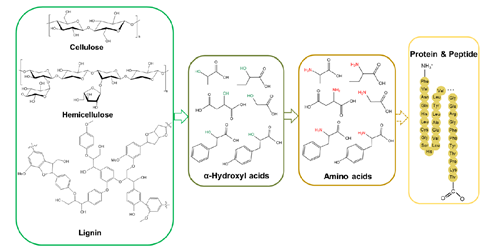
Figure 3. Catalytic transformation of biomass-derived α-hydroxyl acids into amino acids and possible further upgrading into proteins.
Afterward, a bimetallic NiCu/Al2O3 catalyst was developed with further improved catalytic performance. More than 10 alcohols including furfural alcohol, benzyl alcohol, n-propanol, and so forth were converted to nitriles with high to nearly quantitative yields. In addition to amines and nitrile, biomass-derived furfural can be converted into pyrrole heterocyclic chemicals. Furfural is one of the few commercially available platform chemicals from hemicellulose, which serves as a suitable substrate for synthesizing N-heterocyclic compounds (Figure 4a). The combined use of Pd@S-1 and acidic zeolite H-β are the capable catalysts to promote the transformation. A dual-layer fixed-bed reactor (Figure 4b) was designed with two temperature zones optimized, respectively. An overall 75% yield of pyrrole was obtained under the best conditions.
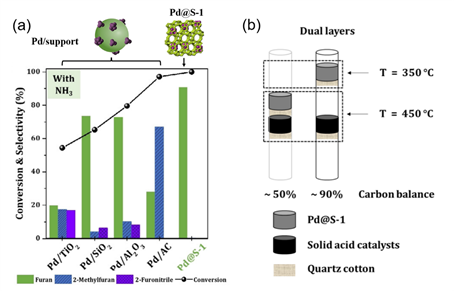
Figure 4. (a) Conversion and product distribution for gas-phase furfural decarbonylation with NH3 by using different Pd catalysts. (b) Dual-layer design of the reactor.
An integrated biorefinery protocol combining shrimp shell waste (SSW) pretreatment and biological fermentation has been established to transform shell waste to tyrosine and L-DOPA (a frontline drug treating Parkinson’s disease) that were previously unavailable from chitin by traditional chemical processes. The pretreatment step was employed with acid to remove minerals in the shell under mild conditions and also to promote chitin and protein hydrolysis under ball mill grinding to water-soluble hydrolysates. Subsequently, the hydrolysates were depolymerized to dimers and monomers using the chitinase enzyme and then fermented with engineered E. coli strains to produce tyrosine and L-DOPA (Figure 5).
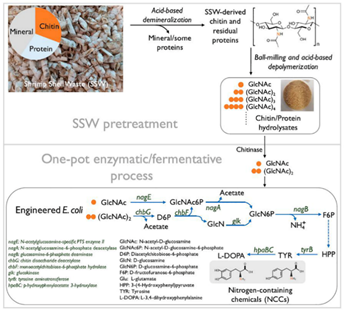
Figure 5. Integrated shell biorefinery process for upcycling SSW-derived chitin
into organonitrogen chemicals.
In the second strategy, chitin can be used as a single feedstock to provide the carbon and nitrogen sources needed for synthesis. The hydrolysis and dehydration of chitin leads to the formation of 3-acetamido-5-acetylfuran (3A5AF), an analog to 5-hydroxymethylfurfural (5-HMF) in cellulose refinery, which then serves as a versatile building block for synthesizing pharmaceuticals, dyes, and functional polymers (Figure 6).
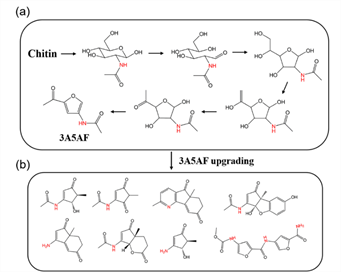
Figure 6. (a) Proposed reaction pathway of chitin conversion to 3A5AF.
(b) Downstream upgrading of 3A5AF to a range of organonitrogen chemicals.
Conclusions
Based on the above comments, it is an important research field to develop the boundary of biomass refining to prepare nitrogen-containing chemicals, which can reduce the consumption of fossil resources, diversify bio-based chemicals and enhance the economic competitiveness of biomass refining. As a new field, there are many challenges and opportunities for biomass preparation of nitrogen-containing chemicals. For example, the development of new efficient catalysts, the combination of artificial intelligence technology and the chemical screenings, and the separation and purification of reaction products need more future research efforts.
Author Introduction
 Xi Chen obtained the Bachelor and Master’s degree in Chemistry at Wuhan University in 2010 and 2012. In 2016, she obtained the PhD from the National University of Singapore under the supervision of Prof Ning Yan and then stayed as a postdoc. In 2018, she joined the China-UK Low Carbon College at Shanghai Jiao Tong University. Her main research focus is on the utilization of chitin and cellulose biomass. At present, she has published more than 30 SCI papers with the total citation > 2000 (data source: Scopus, H-index 21).
Xi Chen obtained the Bachelor and Master’s degree in Chemistry at Wuhan University in 2010 and 2012. In 2016, she obtained the PhD from the National University of Singapore under the supervision of Prof Ning Yan and then stayed as a postdoc. In 2018, she joined the China-UK Low Carbon College at Shanghai Jiao Tong University. Her main research focus is on the utilization of chitin and cellulose biomass. At present, she has published more than 30 SCI papers with the total citation > 2000 (data source: Scopus, H-index 21).
Paper links
https://pubs.acs.org/doi/10.1021/acs.accounts.0c00842?ref=pdf&
LCC Delegation Visits the UK and France
2025-12-16